HYALEX® Knee Cartilage System Clinical Studies
Are you suffering from knee pain?
Hyalex Orthopaedics is conducting two prospective, multi-center, single-arm clinical studies of the HYALEX® Knee Cartilage System in the US and Europe.


Cartilage damage causes mobility-limiting pain and inflammation of the knee joint
Cartilage lesions can result from traumatic injury, overuse, or degenerative osteoarthritis. In younger patients, current treatment is often focused on biologic therapies that attempt to regrow cartilage, and in older patients, total joint replacements using traditional metal-based orthopaedic materials are common.
Hyalex Orthopaedics aims to provide a new solution for cartilage patients using our revolutionary HYALEX® Cartilage platform
The purpose of these clinical trials is to evaluate the safety and technical performance of the HYALEX® Knee Cartilage System.
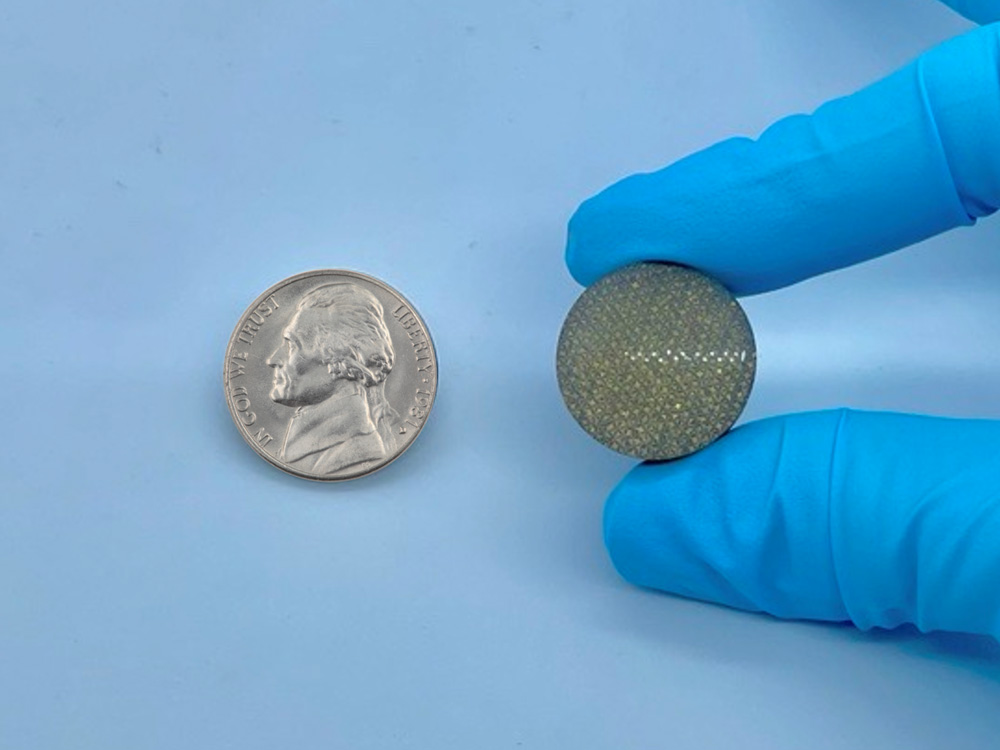
All enrolled patients will receive a HYALEX® Knee Implant, which is composed of our proprietary synthetic HYALEX® Cartilage material bonded to medical-grade porous titanium. The sterile implant is designed to replace painful cartilage lesions and provide immediate restoration of the knee surface.

Am I eligible for the Hyalex clinical trials?
The studies are designed for symptomatic patients who require surgical treatment due to loss of articular cartilage in the knee femoral condyle. The unique design of the HYALEX® Knee Cartilage System allows surgeons to treat patients with or without bony involvement. Please visit the sites below for the full list of eligibility criteria.
- 21 – 65 years old
- Body Mass Index (BMI) ≤ 35
- KOOS pain score between 20 and 65
- Failure of non-operative treatment (e.g., physical therapy) for at least 4 weeks
Our clinical sites in the United States are currently enrolling patients.
Our clinical sites in Europe are currently enrolling patients.